Work and energy have the same units: the joule. Joules are a derived unit of measure in the SI system, being comprised of kg⋅m²/s². Why do we have two different terms for the same thing? What’s the difference?
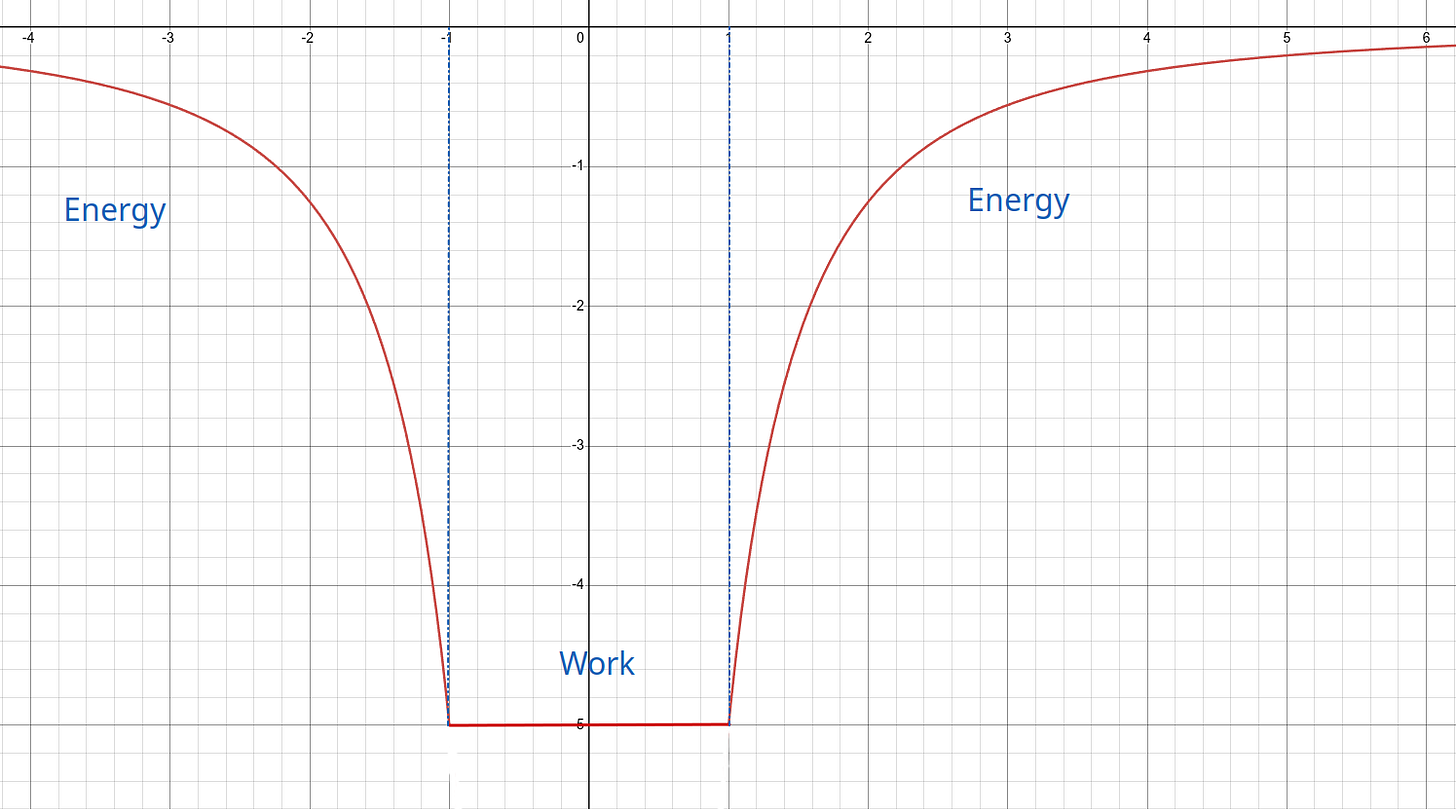
Work is bound. It’s being used. It exists in actuality.
Energy is free. It’s available to be used. It exists in potential.
Work is change. Energy is the ability to cause change.
Work deforms the field. Energy is the deformation of the field.
Here’s one of the secrets of science: That free, potential energy? It never gets used up. It’s always there, whether you use it or not, because of the nature of the field. That’s not to say that whatever’s using the potential energy won’t have an effect on the source doing all the work. It probably will. All those gravitational waves LIGO has detected? Their passages through the earth, each energetic enough to squeeze reality for a brief moment, didn’t use up any of their energy at all. The earth and the moon have held each other in a gravitational embrace for billions of years, at no cost in energy. The atoms in my desk hold up the monitor and keyboard through electromagnetic repulsion, fighting against the gravitic attraction of the entire earth at every moment, at no cost in energy.
If energy actually got used up, all the matter in the universe would have evaporated billions of years ago.
Energy and work are not conserved. They are continuously recreated. Energy density, on the other hand, is strictly conserved. You can’t ever withdraw more energy than the universe provides, and you can’t use less than nothing. The potential energy field pictured above shows zero energy at the top. It has a bottom, far, far below, at some unimaginable yet finite amount of energy. You can’t go above the top. You can’t go below the bottom. You must color within the lines.
No comments:
Post a Comment
I reserve the right to remove egregiously profane or abusive comments, spam, and anything else that really annoys me. Feel free to agree or disagree, but let's keep this reasonably civil.